
Job Opening
We are looking for an innovative, talented and creative Software Engineer to join our team.

We are looking for an innovative, talented and creative Software Engineer to join our team.

QMISG Education organized a seminar on “Malformations of Cortical Development: Latest on Genetics and Imaging” by Alireza Radmanesh, Assistant Professor,
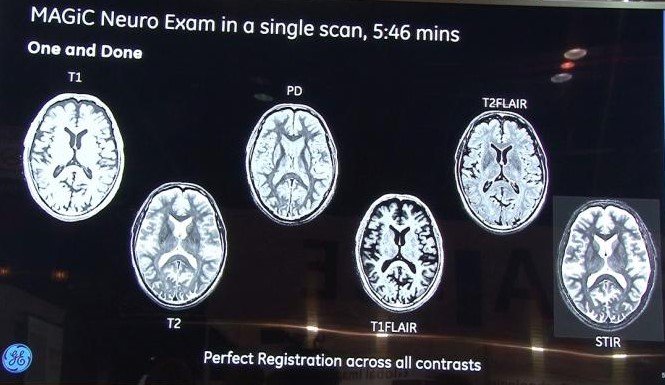
The U.S. Food and Drug Administration (FDA) granted market clearance for GE Healthcare’s MAGiC (MAGnetic resonance image Compilation) software, the
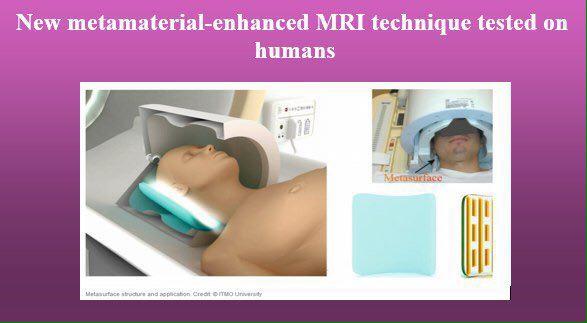
New Metamaterial-Enhanced MRI Technique Scientists from Leiden University Medical Center in the Netherlands and ITMO University in Russia have designed
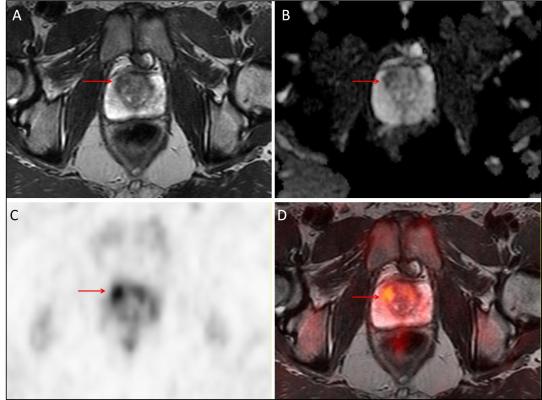
Adding F-18-choline positron emission tomography (PET) to multi-parametric prostate magnetic resonance imaging (mpMRI) improves identification of significant prostate cancer over

We are looking for an innovative, talented and creative Software Engineer to join our team.

QMISG Education organized a seminar on “Malformations of Cortical Development: Latest on Genetics and Imaging”

The U.S. Food and Drug Administration (FDA) granted market clearance for GE Healthcare’s MAGiC (MAGnetic

New Metamaterial-Enhanced MRI Technique Scientists from Leiden University Medical Center in the Netherlands and ITMO

Adding F-18-choline positron emission tomography (PET) to multi-parametric prostate magnetic resonance imaging (mpMRI) improves identification